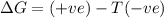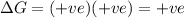Acertain process has δh° > 0, δs° < 0, and δg° > 0. the values of δh° and δs° do not depend on the temperature. which of the following is a correct conclusion about this process? none of the above conclusions is correct. it is non-spontaneous at all t. it is spontaneous at low t. it is spontaneous at all t. it is spontaneous at high t

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:00
One of the few xenon compounds that form is cesium xenon heptafluoride (csxef7). how many moles of csxef7 can be produced from the reaction of 13.0 mol cesium fluoride with 12.5 mol xenon hexafluoride? csf(s) + xef6(s) csxef7(s)
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1

Chemistry, 22.06.2019 20:00
The volume of a single vanadium atom is 9.29×10-24 cm3. what is the volume of a vanadium atom in microliters?
Answers: 3

Chemistry, 22.06.2019 21:00
Use the measurements in the table to determine which unidentified metal has the highest density. metal volume mass a 10.5 cm3 122 g b 14.2 cm3 132 g c 16.1 cm3 115 g d 12.7 cm3 126 g
Answers: 2
You know the right answer?
Acertain process has δh° > 0, δs° < 0, and δg° > 0. the values of δh° and δs° do not dep...
Questions


History, 25.06.2019 05:00







Mathematics, 25.06.2019 05:00


Mathematics, 25.06.2019 05:00




Mathematics, 25.06.2019 05:00



Social Studies, 25.06.2019 05:00


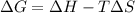
 = Gibbs free energy = +ve
= Gibbs free energy = +ve
 = enthalpy change = +ve
= enthalpy change = +ve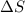 = entropy change = -ve
= entropy change = -ve