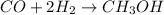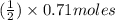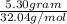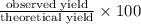Chemistry, 27.12.2019 05:31 lorelei7668
Methanol (ch3oh) can be produced by the following reaction: co(g) 1 2h2(g) 88n ch3oh(g) hydrogen at stp flows into a reactor at a rate of 16.0 l/min. carbon monoxide at stp flows into the reactor at a rate of 25.0 l/min. if 5.30 g of methanol is produced per minute, what is the percent yield of the reaction?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:00
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2

Chemistry, 22.06.2019 06:20
If i can still dissolve more sugar into the solution at a certain temperature what would i call that solution
Answers: 3

Chemistry, 22.06.2019 06:40
Ted and emily played a mixed doubles tennis match against jack and brenda. in the second match. ted and brenda played against jack and emily. which type of chemical reaction does the situation demonstrate?
Answers: 3

Chemistry, 22.06.2019 09:30
Mike and mitchell decide to have a foot race. they mark off a stretch of 100 yards, and recruit cindy to work the stopwatch. after running the race and looking at the results, cindy declared that mitchell was the fastest. so how did the boys times compare?
Answers: 3
You know the right answer?
Methanol (ch3oh) can be produced by the following reaction: co(g) 1 2h2(g) 88n ch3oh(g) hydrogen at...
Questions



SAT, 09.02.2022 15:10


Mathematics, 09.02.2022 15:10

Mathematics, 09.02.2022 15:10

Mathematics, 09.02.2022 15:10


History, 09.02.2022 15:10



Chemistry, 09.02.2022 15:10

Mathematics, 09.02.2022 15:10







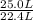 per minute
per minute
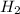 being fed per minute =
being fed per minute = 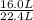 per minute
per minute