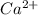ca2+

Chemistry, 21.12.2019 07:31 4804397217
Which ion was formed by providing the second ionization energy to remove an electron?
ca2+
n3–
fe3+
s2–

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:30
According to periodic trend, which of the following most likely has the highest ionization energy? kr be ni sc
Answers: 3


Chemistry, 22.06.2019 14:10
13. a covalent bond between two atoms is likely to be polar if: a. one of the atoms is much more electronegative than the other. b. the two atoms are equally electronegative. c. the two atoms are of the same element. d. the bond is part of a tetrahedrally shaped molecule. e. one atom is an anion.
Answers: 1

Chemistry, 22.06.2019 15:30
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3
You know the right answer?
Which ion was formed by providing the second ionization energy to remove an electron?
ca2+
ca2+
Questions


Arts, 26.01.2021 20:20



Physics, 26.01.2021 20:20

Mathematics, 26.01.2021 20:20



Business, 26.01.2021 20:20


Mathematics, 26.01.2021 20:20


Mathematics, 26.01.2021 20:20




Mathematics, 26.01.2021 20:20

Chemistry, 26.01.2021 20:20


Mathematics, 26.01.2021 20:20