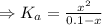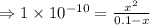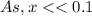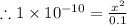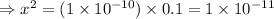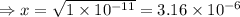Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 14:30
Chemistry worksheet - i am not sure what they are asking for exactly?
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 20:00
Many free radicals combine to form molecules that do not contain any unpaired electrons. the driving force for the radical–radical combination reaction is the formation of a new electron‑pair bond. consider the chemical equation. n(g)+no(g)⟶nno(g) n(g)+no(g)⟶nno(g) write lewis formulas for the reactant and product species in the chemical equation. include nonbonding electrons. n(g)n(g) select draw rings more erase select draw rings more erase select draw rings more erase n no(g)
Answers: 1

Chemistry, 23.06.2019 02:20
Why dose heating increase the speed at which a solution dissolved in water
Answers: 1
You know the right answer?
Bh+clo4- is a salt formed from the base b (kb = 1.00e-4) and perchloric acid. it dissociates into bh...
Questions




Spanish, 25.02.2021 20:20

Health, 25.02.2021 20:20


English, 25.02.2021 20:20

Biology, 25.02.2021 20:20

Mathematics, 25.02.2021 20:20


Mathematics, 25.02.2021 20:20

History, 25.02.2021 20:20

Mathematics, 25.02.2021 20:20


English, 25.02.2021 20:20


History, 25.02.2021 20:20

Mathematics, 25.02.2021 20:20

Mathematics, 25.02.2021 20:20

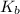 = 1 × 10⁻⁴, Concentration of salt: BH⁺ClO₄⁻ = 0.1 M
= 1 × 10⁻⁴, Concentration of salt: BH⁺ClO₄⁻ = 0.1 M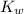 = 1 × 10⁻¹⁴
= 1 × 10⁻¹⁴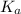 ) for the weak acid (BH⁺) can be calculated by the equation:
) for the weak acid (BH⁺) can be calculated by the equation: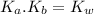
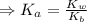
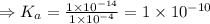
![K_{a} = \frac{\left [B \right ] \left [H_{3}O^{+}\right ]}{\left [BH^{+} \right ]} = \frac{(x)(x)}{(0.1 - x)} = \frac{x^{2}}{0.1 - x}](/tpl/images/0434/5359/b792d.png)