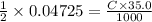We find that 18.90 milliliters of a 2.50m koh solution are required to titrate 35.0 milliters of a h2s04 solution. what is the molarity of the h2s04 solution? 2 2koh + h2s04 k2s04 2 h20 a. 0.675 m b. 1.57 m c. 0.0711 m d. 0.128 m e. 4.04 m f. 0.903 m g. 1.18 m h. 0.0173 m

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 06:00
If you burn 10 kilograms of wood in a fire (combustion) what is the weight of the products after the fire has finished burning the wood?
Answers: 3

Chemistry, 22.06.2019 12:50
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1

You know the right answer?
We find that 18.90 milliliters of a 2.50m koh solution are required to titrate 35.0 milliters of a h...
Questions

Social Studies, 15.02.2021 14:40

Mathematics, 15.02.2021 14:40


Biology, 15.02.2021 14:40

Mathematics, 15.02.2021 14:40



Physics, 15.02.2021 14:40

Mathematics, 15.02.2021 14:40

Biology, 15.02.2021 14:40




Mathematics, 15.02.2021 14:40

English, 15.02.2021 14:40

English, 15.02.2021 14:40

Mathematics, 15.02.2021 14:40

Geography, 15.02.2021 14:40

Mathematics, 15.02.2021 14:40

History, 15.02.2021 14:50

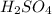 is 0.675 M
is 0.675 M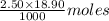 of KOH = 0.04725 moles of KOH
of KOH = 0.04725 moles of KOH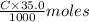 of
of