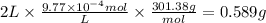Chemistry, 28.12.2019 01:31 ronniethefun
The drug dobutamine (fw=301.38g/mol, c18h23no3c18h23n03) has a molar absorptivity of 703 at 262nm. one tablet is dissolved in water and diluted to a volume of 2l. if the solution exhibits an absorbance of 0.687 in the uv region at 262nm in a 1- cm cell, how many grams to dobutamine are contained in the tablet? a. 0.5277g b. 0.58889 c. 9.77x 10g d. 97.778ge. 9.7778g

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:00
City a and city b had two different temperatures on a particular day. on that day, four times the temperature of city a was 8â° c more than 3 times the temperature of city b. the temperature of city a minus twice the temperature of city b was â’3â° c. what was the temperature of city a and city b on that day? city a was 5â° c, and city b was 4â° c. city a was 3â° c, and city b was â’1â° c. city a was 8â° c, and city b was â’3â° c. city a was 5â° c, and city b was â’5â° c.
Answers: 2

Chemistry, 22.06.2019 01:30
Agas is contained in a thick walled balloon when the pressure changes from 1.21 atm to 2.52 the volume changes from 3.75 l to 1.72 l and the temperature change from 293k to blank k
Answers: 3

Chemistry, 22.06.2019 03:30
Adrop of acetone (nail polish remover) has a mass of 35 mg and a density of 0.788 g/cm3. what is its volume in cubic centimeters?
Answers: 3

Chemistry, 22.06.2019 12:00
the mississippians were considered to be horticulturalists, which means they were
Answers: 1
You know the right answer?
The drug dobutamine (fw=301.38g/mol, c18h23no3c18h23n03) has a molar absorptivity of 703 at 262nm. o...
Questions


History, 14.10.2019 03:30

Mathematics, 14.10.2019 03:30


History, 14.10.2019 03:30

Chemistry, 14.10.2019 03:30


History, 14.10.2019 03:30




Mathematics, 14.10.2019 03:30


Mathematics, 14.10.2019 03:30


History, 14.10.2019 03:30


Mathematics, 14.10.2019 03:30

Social Studies, 14.10.2019 03:30

Biology, 14.10.2019 03:30