
Chemistry, 28.12.2019 02:31 Mercedes12152002
For the reaction x + y → z, the reaction rate is found to depend only upon the concentration of x. a plot of 1/x verses time gives a straight line. picture what is the rate law for this reaction?
a. rate = k [x]
b. rate = k [x]2
c. rate = k [x][y]
d. rate = k [x]2[y]

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:30
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2

Chemistry, 22.06.2019 20:20
The characteristics of two different types of reactions are shown below: reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of an element. which statement is true about the atoms of the elements that participate in the two reactions? their identity changes in both reaction a and reaction b. their identity changes in reaction a but not in reaction b. their identity changes in reaction b but not in reaction a. their identity remains the same in both reaction a and reaction b.
Answers: 1


Chemistry, 23.06.2019 09:00
Which question could be best answered using the process of scientific inquiry? do different plates have different rock compositions? why did it take so long to develop the theory of plate tectonics? what are different cultural myths caused by plate tectonics? do plates move intentionally to cause volcanic eruptions?
Answers: 3
You know the right answer?
For the reaction x + y → z, the reaction rate is found to depend only upon the concentration of x. a...
Questions

Mathematics, 14.12.2020 14:00

Computers and Technology, 14.12.2020 14:00

Mathematics, 14.12.2020 14:00

History, 14.12.2020 14:00

History, 14.12.2020 14:00

Chemistry, 14.12.2020 14:00

Mathematics, 14.12.2020 14:00

Mathematics, 14.12.2020 14:00




Chemistry, 14.12.2020 14:00

Mathematics, 14.12.2020 14:00

English, 14.12.2020 14:00

Mathematics, 14.12.2020 14:00



Chemistry, 14.12.2020 14:00


Mathematics, 14.12.2020 14:00

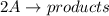
![rate = k[A]^{2}](/tpl/images/0435/2384/bea1f.png) , where k is rate constant and [A] is concentration of A
, where k is rate constant and [A] is concentration of A![\frac{1}{[A]}=\frac{1}{[A]_{0}}+kt](/tpl/images/0435/2384/a6d73.png) , where
, where ![[A]_{0}](/tpl/images/0435/2384/48818.png) is initial concentration of reactant A and [A] is concentration of A after "t" time (k and [A]_{0}[/tex] are constants)
is initial concentration of reactant A and [A] is concentration of A after "t" time (k and [A]_{0}[/tex] are constants)![\frac{1}{[A]}](/tpl/images/0435/2384/153b1.png) is y and t is x.
is y and t is x.![rate = k[X]^{2}](/tpl/images/0435/2384/7b96c.png)


