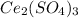Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:40
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2

Chemistry, 22.06.2019 17:20
Which of these features are formed when hot groundwater is forced out through cracks in the earth's surface?
Answers: 2


Chemistry, 22.06.2019 23:00
What is the name of the enzyme that forms at the start of transcription?
Answers: 1
You know the right answer?
Substances with high lattice energies tend to be less soluble than substances with low lattice energ...
Questions


Mathematics, 19.03.2021 18:10

History, 19.03.2021 18:10


Mathematics, 19.03.2021 18:10


Health, 19.03.2021 18:10


Mathematics, 19.03.2021 18:10

Mathematics, 19.03.2021 18:10

History, 19.03.2021 18:10






Mathematics, 19.03.2021 18:10

Mathematics, 19.03.2021 18:10


 >
>