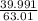Asolution is prepared by dissolving 5g hno3 in pure water to a total volume of 1l.
a) what is the ph
b) what mass of strong base sodium hydroxide (naoh) would be required to neutralize the solution?
c) if the sodium hydroxide is available as a .1m solution, what volume of the basic solution will be needed to neutralize the acid?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:10
What does a particular point on a line of a phase diagram represent? o a. the maximum temperature a substance can exist at without bonds breaking b. the pressure created by the kinetic energy of molecules at a particular temperature c. the melting point or boiling point of a substance at a specific pressure d. the conditions in which temperature and pressure have equal effects on a substance
Answers: 2

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be changed varied experimented controlled
Answers: 1

Chemistry, 22.06.2019 13:30
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1

Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1
You know the right answer?
Asolution is prepared by dissolving 5g hno3 in pure water to a total volume of 1l.
a) w...
a) w...
Questions

History, 09.12.2020 01:00



Mathematics, 09.12.2020 01:00


English, 09.12.2020 01:00










Arts, 09.12.2020 01:00

English, 09.12.2020 01:00

Mathematics, 09.12.2020 01:00

History, 09.12.2020 01:00

Mathematics, 09.12.2020 01:00