
At equilibrium, the concentrations of the products and reactants for the reaction, h2 (g) + i2 (g) 2 hi (g), are [h2] = 0.106 m; [i2] = 0.022 m; [hi] = 1.29 m calculate the new equilibrium concentration of hi (in m) if the equilibrium concentrations of h2 and i2 are 0.95 m and 0.019 m respectively.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:30
In numbering carbon atoms in the parent chain of a hydrocarbon, why would you number from right to left, rather than left to right
Answers: 1

Chemistry, 22.06.2019 17:00
In a heat engine of 1000 j of heat enters the system and the piston does 500 j of work what is the final internal energy of the system if the inital energy was 2000 j we have to do all of these down here 1)write the equation 2)list out your know variables 3)plug the numbers into the equations 4)solve 5)write your solution statemtn that includes inital energuy and final energuy added
Answers: 1

Chemistry, 22.06.2019 18:00
The fact that the total amount of energy in a system remains constant is a(n)
Answers: 1

Chemistry, 23.06.2019 02:50
What is the typical rotational frequency frot for a molecule like n2 at room temperature (25∘c)? assume that d for this molecule is 1å=10−10m. take the total mass of an n2 molecule to be mn2=4.65×10−26kg. you will need to account for rotations around two axes (not just one) to find the correct frequency. express frot numerically in hertz, to three significant figures.
Answers: 3
You know the right answer?
At equilibrium, the concentrations of the products and reactants for the reaction, h2 (g) + i2 (g) ...
Questions




English, 19.11.2020 18:50

Mathematics, 19.11.2020 18:50


Mathematics, 19.11.2020 18:50

Geography, 19.11.2020 18:50



Mathematics, 19.11.2020 18:50




Mathematics, 19.11.2020 18:50





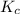 ) for the given chemical reaction, is given by the equation:
) for the given chemical reaction, is given by the equation:![K_{c} = \frac {[HI]^{2}}{[H_{2}]\: [I_{2}]}](/tpl/images/0435/6174/f8601.png)
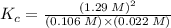
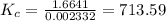
![K_{c} = \frac {[HI]^{2}}{(0.95\: M) \times (0.019\: M)}](/tpl/images/0435/6174/d2511.png)
![\Rightarrow K_{c} = 713.59 = \frac {[HI]^{2}}{0.01805}](/tpl/images/0435/6174/fef3c.png)
![\Rightarrow [HI]^{2} = 713.59 \times 0.01805 = 12.88](/tpl/images/0435/6174/d4ab1.png)
![\Rightarrow [HI] = \sqrt {12.88} = 3.589 M](/tpl/images/0435/6174/5120a.png)


