Which of the following bets explains why the bond in ammonia nh3 are polar covalent?
...

Chemistry, 29.12.2019 23:31 natalie2sheffield
Which of the following bets explains why the bond in ammonia nh3 are polar covalent?
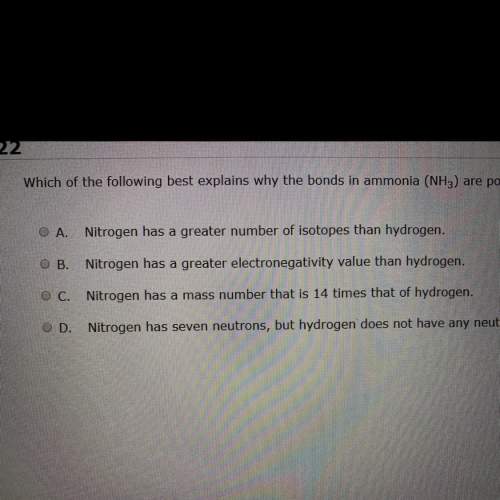

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 14:00
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3

Chemistry, 23.06.2019 03:50
Which best describes the activation energy of a chemical reaction? a. the combined energy of all the reactants b. the amount of energy required for a reaction to occur c. the difference in energy between products and reactants d. the potential energy stored in the bonds of reactants and products
Answers: 1

Chemistry, 23.06.2019 11:00
Achemist weighed out 101.g of silver. calculate the number of moles of silver she weighed out.
Answers: 2

Chemistry, 23.06.2019 15:30
K12 chemistry unit assessment: chemical bonding, electrostatic forces in ionic bonds hold which of the following together? 1. he atoms in helium gas 2.na+ and br- in nabr 3. fe atoms and localized electrons in iron
Answers: 1
You know the right answer?
Questions







Mathematics, 09.07.2019 12:00










History, 09.07.2019 12:00

Computers and Technology, 09.07.2019 12:00

Mathematics, 09.07.2019 12:00




