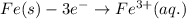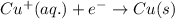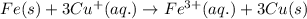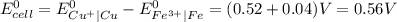Determine the overall reaction and its standard cell potential (in v) at 25°c for the reaction involving the galvanic cell made from a half-cell consisting of a copper electrode in 1 m copper(i) nitrate solution and a half-cell consisting of an iron electrode in 1 m iron(iii) nitrate. (include states-of-matter under the given conditions in your answer. use the lowest possible whole number coefficients.) overall reaction

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 23:30
Start an single atom tab. observe the decay of polonium-211. after each decay, press the reset nucleus button to watch the process again. write a description of alpha decay for po-211
Answers: 2

Chemistry, 22.06.2019 05:30
What happens to the atomic radius when an elctron is lost
Answers: 1

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be changed varied experimented controlled
Answers: 1

Chemistry, 22.06.2019 16:10
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2
You know the right answer?
Determine the overall reaction and its standard cell potential (in v) at 25°c for the reaction invol...
Questions

Biology, 10.11.2020 20:40


Mathematics, 10.11.2020 20:40


Mathematics, 10.11.2020 20:40

Mathematics, 10.11.2020 20:40


Biology, 10.11.2020 20:40

Mathematics, 10.11.2020 20:40




History, 10.11.2020 20:40

Mathematics, 10.11.2020 20:40

Chemistry, 10.11.2020 20:40


English, 10.11.2020 20:40

Chemistry, 10.11.2020 20:40


Biology, 10.11.2020 20:40

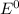 ) are given below-
) are given below-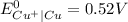
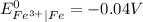
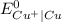 is greater than
is greater than 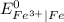 therefore Fe will be oxidized to
therefore Fe will be oxidized to 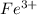 and
and 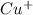 will be reduced to Cu
will be reduced to Cu