
Astudent is doing a titration using potassium permanganate solution, kmno4, to determine the amount of h2o2 in a sample. the balanced equation for the reaction in the titration is given below:
2 mno4-(aq) + 6 h+(aq) + 5 h2o2(aq) --> ž 2 mn2+(aq) + 8 h2o(l) + 5 o2(g)
a student calculates an amount of moles of h2o2 that is larger than the actual value. which of the following errors could correctly explain the larger value?
the student failed to wear goggles.
the student did not swirl the flask appropriately and therefore stopped short of the endpoint.
the student failed to rinse the buret with kmno4¬ solution after rinsing it with distilled water.
the student added an extra 15 ml of distilled water to the h2o2 solution.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 1

Chemistry, 22.06.2019 10:30
How do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 2

Chemistry, 22.06.2019 12:00
Hey guys so i need to know what is _nh3+> nh4oh ~chemistry~
Answers: 1

You know the right answer?
Astudent is doing a titration using potassium permanganate solution, kmno4, to determine the amount...
Questions




Social Studies, 05.10.2021 23:30



Mathematics, 05.10.2021 23:30




Mathematics, 05.10.2021 23:30



English, 05.10.2021 23:30




Mathematics, 05.10.2021 23:30

Biology, 05.10.2021 23:40

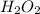 solution.
solution.

