
Chemistry, 30.12.2019 23:31 6224968918
Outline the steps needed to determine the limiting reactant when 30.0 g of propane, c3h8, is burned with 75.0 g of oxygen.
percent yield = 0.8347g / 0.9525 g × 100% = 87.6%

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:00
What mass of carbon dioxide is produced from the complete combustion of 4.50×10−3 g of methane?
Answers: 2

Chemistry, 22.06.2019 12:00
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1

Chemistry, 23.06.2019 07:00
Ajar contains a certain substance. which observation would show that the substance must be either a solid or a liquid?
Answers: 1

Chemistry, 23.06.2019 08:10
Time remaining 58: 10 an atom that has 84 protons and 86 neutrons undergoes a reaction. at the end of the reaction, it has 82 protons and 84 neutrons. what happened to the atom? it accepted radiation in a chemical reaction it donated neutrons to another atom in a chemical reaction it emitted an alpha particle in a nuclear reaction. it accepted protons in a nuclear reaction. mark this and retum save and exit next submit
Answers: 3
You know the right answer?
Outline the steps needed to determine the limiting reactant when 30.0 g of propane, c3h8, is burned...
Questions

Mathematics, 26.02.2021 21:00

History, 26.02.2021 21:00

Mathematics, 26.02.2021 21:00

English, 26.02.2021 21:00





Mathematics, 26.02.2021 21:00



Mathematics, 26.02.2021 21:00

Health, 26.02.2021 21:00

Mathematics, 26.02.2021 21:00

Chemistry, 26.02.2021 21:00



Mathematics, 26.02.2021 21:00


Mathematics, 26.02.2021 21:00

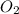
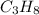 = 30.0 g
= 30.0 g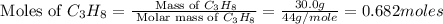
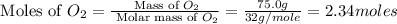
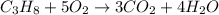
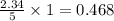 moles of
moles of 

