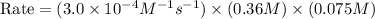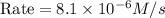Chemistry, 31.12.2019 00:31 texas101st78
The rate constant for the reaction nh4+(aq) + no2−(aq) --> n2(g) + 2h2o(l) is, k = 3.0 × 10^−4 m^-1 · s^-1. calculate the rate of the reaction if [nh4+] = 0.36 m and [no2−] = 0.075 m. (the reaction is in first order in regards to nh4+ as well as no2-) answer in scientific notation

Answers: 3
Another question on Chemistry



Chemistry, 22.06.2019 23:30
Substance a is a nonpolar liquid and has only dispersion forces among its constituent particles. substance b is also a nonpolar liquid and has about the same magnitude of dispersion forces among its constituent particles. when substance a and b are combined, they spontaneously mix.
Answers: 1

Chemistry, 23.06.2019 00:30
What is calcium oxide+diphosphorus pentoxide--> calcium phosphate balanced
Answers: 1
You know the right answer?
The rate constant for the reaction nh4+(aq) + no2−(aq) --> n2(g) + 2h2o(l) is, k = 3.0 × 10^−4 m...
Questions


History, 15.10.2020 16:01


Mathematics, 15.10.2020 16:01


Mathematics, 15.10.2020 16:01

Mathematics, 15.10.2020 16:01

Business, 15.10.2020 16:01





Arts, 15.10.2020 16:01



Mathematics, 15.10.2020 16:01

Spanish, 15.10.2020 16:01

Health, 15.10.2020 16:01


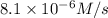
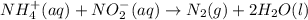
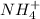 and
and 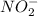 are the reactants.
are the reactants.![\text{Rate}=k[NH_4^+][NO_2^-]](/tpl/images/0437/6898/ed258.png)
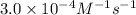
![[NH_4^+]](/tpl/images/0437/6898/5c46c.png) = concentration of
= concentration of ![[NO_2^-]](/tpl/images/0437/6898/10a69.png) = concentration of
= concentration of