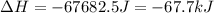Chemistry, 31.12.2019 01:31 cearadenney7067
How much energy must be removed from a 125 g sample of benzene (molar mass= 78.11 g/mol) at 425.0 k to liquify the sample and lower the temperature to 335.0 k? the following physical data may be useful.
δhvap = 33.9 kj/mol
δhfus = 9.8 kj/mol
cliq = 1.73 j/g°c
cgas = 1.06 j/g°c
csol = 1.51 j/g°c
tmelting = 279.0 k
tboiling = 353.0 k

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 13:00
Which organic molecule is found in the chromatin of cells?
Answers: 1

Chemistry, 22.06.2019 10:20
Gwhich r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? which r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? −ch2−oh −ch2−o||c−nh2 −ch2−coo− −ch2−ch2−ch2−ch2−n+h3
Answers: 3

Chemistry, 22.06.2019 14:50
How are evaporation and sublimation similar? a both involve the formation of a gas. b both release energy to the surroundings. c both take place throughout a solid. d both take place at the surface of a liquid.
Answers: 1

Chemistry, 22.06.2019 21:30
An atomic nucleus is composed ofa)protons.b)protons and neutrons.c)protons and electrons.d)protons, neutrons, and electrons.
Answers: 1
You know the right answer?
How much energy must be removed from a 125 g sample of benzene (molar mass= 78.11 g/mol) at 425.0 k...
Questions



English, 14.12.2019 18:31


Social Studies, 14.12.2019 18:31


Chemistry, 14.12.2019 18:31

History, 14.12.2019 18:31

Physics, 14.12.2019 18:31


Mathematics, 14.12.2019 18:31





Mathematics, 14.12.2019 18:31

Mathematics, 14.12.2019 18:31



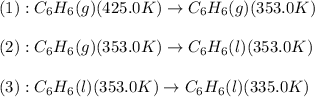
![\Delta H=[m\times c_{p,g}\times (T_{final}-T_{initial})]+m\times \Delta H_{vap}+[m\times c_{p,l}\times (T_{final}-T_{initial})]](/tpl/images/0437/7629/fdeb2.png)
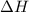 = heat released by the reaction = ?
= heat released by the reaction = ?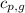 = specific heat of gaseous benzene =
= specific heat of gaseous benzene = 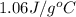
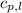 = specific heat of liquid benzene =
= specific heat of liquid benzene = 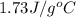
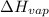 = enthalpy change for vaporization =
= enthalpy change for vaporization = 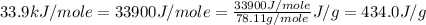
![\Delta H=[125g\times 1.06J/g.K\times (353.0-(425.0))K]+125g\times -434.0J/g+[125g\times 1.73J/g.K\times (335.0-353.0)K]](/tpl/images/0437/7629/08ed2.png)