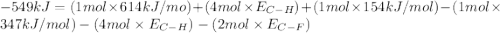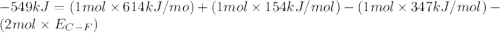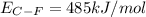Chemistry, 31.12.2019 02:31 yazanadel56
Consider the following reaction: c2h4(g) + f2(g) > c2h4f2(g) delta h = -549 kjestimate the carbon-fluorine bond energy given that the c-c bond energy is 347 kj/mol, the c=c bond energy is 614 kj/mol, and the f-f bond energy is 154 kj/mol.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 20:30
The balanced chemical equation for this lab is: 3cucl2(aq) + 2al(s) 3cu(s) + 2alcl3(aq) if 10.5 g copper chloride react with 12.4 g aluminum, what is the limiting reactant?
Answers: 3

Chemistry, 21.06.2019 21:00
What pressure will be exerted by 0.675 moles of a gas at 25*c if it is in a 0.750-l container?
Answers: 1


Chemistry, 22.06.2019 09:00
Plz mark brainliest 30 points1) find the momentum of a 12 kg snowball that is rolling with a velocity of 9 m/s.2) an 8 ball with a mass of .5 kg is sitting at rest. it is hit by the cue ball (1 kg) traveling at 2.5 m/s. if the cue ball is at rest after the collision, how fast is the 8 ball traveling after the collision? 3) two football players are running toward each other. if the offensive player is 75 kg and is running 8 m/s, how fast must the 60 kg defensive player run in order for the two players to hit and stop?
Answers: 1
You know the right answer?
Consider the following reaction: c2h4(g) + f2(g) > c2h4f2(g) delta h = -549 kjestimate the carbo...
Questions

Chemistry, 29.01.2021 09:30


Mathematics, 29.01.2021 09:30

English, 29.01.2021 09:30

Mathematics, 29.01.2021 09:30

Mathematics, 29.01.2021 09:30

English, 29.01.2021 09:30


Mathematics, 29.01.2021 09:30



Mathematics, 29.01.2021 09:30

Mathematics, 29.01.2021 09:30


Mathematics, 29.01.2021 09:30



Mathematics, 29.01.2021 09:30


Mathematics, 29.01.2021 09:30

![\Delta H_{rxn}=\sum [n_{i}\times (E_{bond})_{i}]-\sum [n_{j}\times (E_{bond})_{j}]](/tpl/images/0437/8404/d74f8.png)
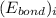 and
and 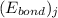 represents average bond energy in breaking "i" th bond and forming "j" th bond respectively.
represents average bond energy in breaking "i" th bond and forming "j" th bond respectively.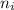 and
and 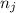 are number of moles of bond break and form respectively.
are number of moles of bond break and form respectively.