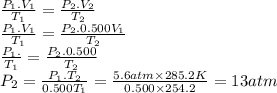Chemistry, 31.12.2019 03:31 payshencec21
For many purposes we can treat methane as an ideal gas at temperatures above its boiling point of . suppose the temperature of a sample of methane gas is lowered from to , and at the same time the pressure is changed. if the initial pressure was and the volume increased by , what is the final pressure? round your answer to significant digits.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
In order to calculate the amount of heat transferred you must know the __ and specific heat of the material, as well as the change in temperature. a. volume b. density c. mass d. enthalpy
Answers: 1


Chemistry, 22.06.2019 17:30
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2

Chemistry, 23.06.2019 10:30
When a chemist collects hydrogen gas over water, she ends up with a mixture of hydrogen and water vapor in her collecting bottle if the pressure in the collecting bottle is 97.1 kilopascals and the vapor pressure of the water is 3 2 kilopascals, what is the partial pressure of the hydrogen?
Answers: 1
You know the right answer?
For many purposes we can treat methane as an ideal gas at temperatures above its boiling point of ....
Questions


Biology, 25.07.2019 13:00





Business, 25.07.2019 13:00

Mathematics, 25.07.2019 13:00

History, 25.07.2019 13:00





History, 25.07.2019 13:00

Mathematics, 25.07.2019 13:00

Chemistry, 25.07.2019 13:00

Mathematics, 25.07.2019 13:00

Mathematics, 25.07.2019 13:00

Social Studies, 25.07.2019 13:00