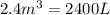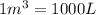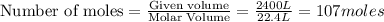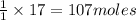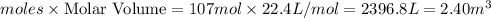Chemistry, 31.12.2019 03:31 kalcius674
Carbon disulfide gas and oxygen gas react to form sulfur dioxide gas and carbon dioxide gas. what volume of carbon dioxide would be produced by this reaction if of carbon disulfide were consumed? also, be sure your answer has a unit symbol, and is rounded to significant digits.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 11:20
Which of the following contributes to the structural rigidity of cellulose? adjacent glucose polymers are stabilized by hydrogen bonding. glucose residues are joined by (α1→4) linkages. cellulose is a highly branched molecule. the conformation of the glucose polymer is a coiled structure.
Answers: 2


Chemistry, 23.06.2019 01:00
Chromium(iii) sulfate is a transition metal compound containing the metal chromium and the polyatomic ion sulfate. the oxidation state of chromium in this compound is , and the chemical formula of the compound is ( ) . reset next
Answers: 3

Chemistry, 23.06.2019 03:50
Show how to convert the temperature 84.7° c to kelvin. include all steps and label the final answer
Answers: 1
You know the right answer?
Carbon disulfide gas and oxygen gas react to form sulfur dioxide gas and carbon dioxide gas. what vo...
Questions



Mathematics, 20.02.2020 23:22


History, 20.02.2020 23:22





Computers and Technology, 20.02.2020 23:23



Chemistry, 20.02.2020 23:23



Mathematics, 20.02.2020 23:23




English, 20.02.2020 23:23

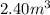
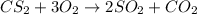
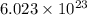 of particles.
of particles.