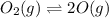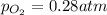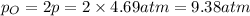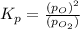Chemistry, 31.12.2019 04:31 angelespinosa521
Asample of o2(g) is placed in an otherwise empty, rigid container at 4224 k at an initial pressure of 4.97 atm, where it decomposes to o(g) by the reaction below.
o2(g) ⇄ 2 o(g)
at equilibrium, the partial pressure of o2 is 0.28 atm. calculate kp for this reaction at 4224 k.

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 1

Chemistry, 22.06.2019 05:50
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1

Chemistry, 22.06.2019 11:00
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3
You know the right answer?
Asample of o2(g) is placed in an otherwise empty, rigid container at 4224 k at an initial pressure o...
Questions

Mathematics, 24.04.2020 11:50





Mathematics, 24.04.2020 11:50

Mathematics, 24.04.2020 11:50

Biology, 24.04.2020 11:50

Physics, 24.04.2020 11:50


Mathematics, 24.04.2020 11:50




Arts, 24.04.2020 11:51


Computers and Technology, 24.04.2020 11:51



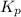 at 4224 K is 314.23.
at 4224 K is 314.23.