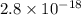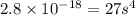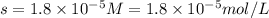Chemistry, 31.12.2019 04:31 babygirl10302015
The solubility product ag3po4 is: ksp = 2.8 x 10^-18. what is the solubility of ag3po4 in water, in moles per liter?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 18:40
Determine the energy released per kilogram of fuel used. given mev per reaction, calculate energy in joules per kilogram of reactants. consider 1 mole of tritium plus 1 mole of deuterium to be a mole of “reactions” (total molar mass = 5 grams).
Answers: 1

Chemistry, 22.06.2019 06:40
Which statement correctly describes metallic bonds? a. they form when certain atoms lose electrons and other atoms gain electrons. b. they involve an attraction between anions and cations. they always involvpoth a metal and a nonmetal. d. they can only form between atoms of the same element. e. they form because electrons can move freely between atoms.
Answers: 3

Chemistry, 22.06.2019 11:00
Which are examples of how technology has advanced scientific understanding.1using hot water to sterilize medical equipment.2transplanting a human organ into another individual.3inserting genes from one sheep into another cell to make a cloneunderstanding the different structures that make up a cell.4examining microorganisms from the deepest parts of the ocean
Answers: 2

You know the right answer?
The solubility product ag3po4 is: ksp = 2.8 x 10^-18. what is the solubility of ag3po4 in water, in...
Questions


Geography, 01.05.2021 17:50




Computers and Technology, 01.05.2021 17:50



Mathematics, 01.05.2021 17:50


Mathematics, 01.05.2021 17:50

Spanish, 01.05.2021 17:50






Mathematics, 01.05.2021 17:50

Biology, 01.05.2021 18:00

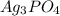 in water is,
in water is, 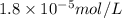
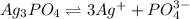
![K_{sp}=[Ag^{+}]^3[PO_4^{3-}]](/tpl/images/0437/9779/7aeef.png)
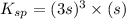
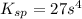
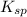 =
=