
The overall reaction 2co3+(aq) + 2cl-(aq) ? 2co2+(aq) + cl2(g) has the standard cell voltage eocell= 0.46 v.
given that cl2(g) + 2e-? 2cl-(aq), eo = 1.36 v,
calculate the standard reduction potential for the following the half reaction at 25oc:
co3+ + e-? co2+
1.82 v
-0.90 v
0.90 v
-1.82 v

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 19:00
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1

Chemistry, 22.06.2019 19:40
What causes different colors to appear in the sky? the absorption of light by air molecules the reflection of light by bodies of water the greenhouse effect in earth's atmosphere the scattering and reflection of light by dust particles
Answers: 2


You know the right answer?
The overall reaction 2co3+(aq) + 2cl-(aq) ? 2co2+(aq) + cl2(g) has the standard cell voltage eocell...
Questions






Mathematics, 23.04.2020 19:35



Mathematics, 23.04.2020 19:35

Chemistry, 23.04.2020 19:35

English, 23.04.2020 19:35


History, 23.04.2020 19:35

Mathematics, 23.04.2020 19:35




Mathematics, 23.04.2020 19:35


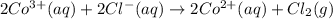
 ,
, 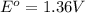
 ,
, 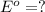
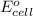 of this reaction is as follows.
of this reaction is as follows.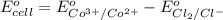
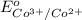 - 1.36 V
- 1.36 V

