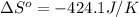Calculate the standard entropy change for the industrial synthesis of urea (a common fertilizer): co2(g) + 2 nh3(g) → co(nh2)2(s) + h2o(ℓ) at 25◦c. patel (smp4358) – 28: fall exam review part three – lyssy – (112476) 8 s ◦ co2(g) 213.74 j k·mol nh3(g) 192.45 j k·mol co(nh2)2(s) 104.6 j k·mol h2o(ℓ) 69.91 j k·mol answer in units of j k · mol.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:30
Select each correct answer. more than one answer may be correct. which of the following is a characteristic of unicellular organisms? they can possess tissues and organs. all of their functions are performed by a single cell. they are usually microscopic. each of their cells is specialized to perform a specific function.
Answers: 1

Chemistry, 22.06.2019 03:10
Between 2014 and 2016, more than 25,000 children in flint, michigan, drank water that was contaminated with lead from lead pipes. during this time, the city claimed the water was safe to drink. which of these actions could the city have taken to ensure that the drinking water was free from lead?
Answers: 3

Chemistry, 22.06.2019 04:30
This question is about electrolysis. metal spoons can be coated with silver. this is called electroplating. suggest one reason why spoons are electroplated?
Answers: 1

Chemistry, 22.06.2019 11:00
Imagine that twenty i.u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2
You know the right answer?
Calculate the standard entropy change for the industrial synthesis of urea (a common fertilizer): c...
Questions



Biology, 06.04.2020 18:25

English, 06.04.2020 18:25



English, 06.04.2020 18:25

History, 06.04.2020 18:25



English, 06.04.2020 18:25


English, 06.04.2020 18:25





History, 06.04.2020 18:25


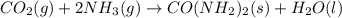
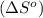 is:
is: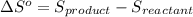
![\Delta S^o=[n_{CO(NH_2)_2}\times \Delta S^0_{(CO(NH_2)_2)}+n_{H_2O}\times \Delta S^0_{(H_2O)}]-[n_{CO_2}\times \Delta S^0_{(CO_2)}+n_{NH_3}\times \Delta S^0_{(NH_3)}]](/tpl/images/0438/1232/f3a9c.png)
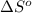 = entropy change of reaction = ?
= entropy change of reaction = ?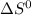 = standard entropy change
= standard entropy change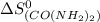 = 104.6 J/mol.K
= 104.6 J/mol.K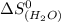 = 69.91 J/mol.K
= 69.91 J/mol.K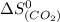 = 213.74 J/mol.K
= 213.74 J/mol.K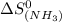 = 192.45 J/mol.K
= 192.45 J/mol.K![\Delta S^o=[1mole\times (104.6J/K.mole)+1mole\times (69.91J/K.mole)}]-[1mole\times (213.74J/K.mole)+2mole\times (192.45J/K.mole)]](/tpl/images/0438/1232/6f6bf.png)