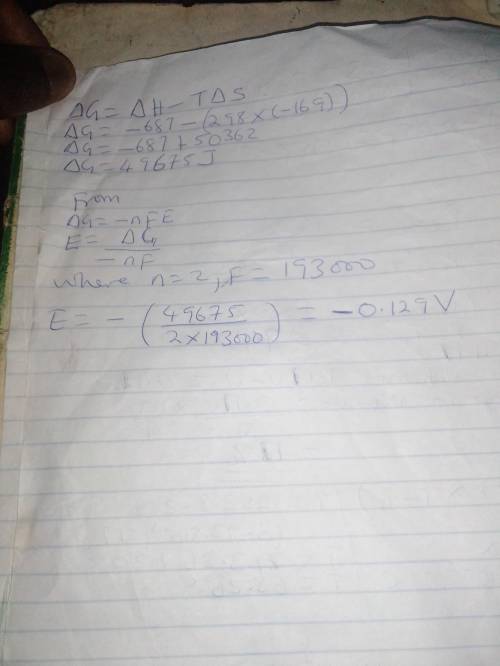Chemistry, 02.01.2020 22:31 Kiaraboyd9366
Calculate the standard cell potential at 25 ∘c for the reaction x(s)+2y+(aq)→x2+(aq)+2y(s) where δh∘ = -687 kj and δs∘ = -169 j/k .
express your answer to three significant figures and include the appropriate

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:30
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. the values of phosphorous acid are 1.30 6.70 calculate the ph for each of the given points in the titration of 50.0 ml of 1.5 m h3po3(aq) with 1.5 m koh(aq) .
Answers: 3

Chemistry, 22.06.2019 07:30
Calculate the ratio of h+ ions to oh– ions at a ph = 7. find the concentration of h+ ions to oh– ions listed in table b of your student guide. then divide the h+ concentration by the oh– concentration. record this calculated ratio in table a of your student guide. compare your approximated and calculated ratios of h+ ions to oh– ions at a ph = 7. are they the same? why or why not? record your comparison in table a. what is the concentration of h+ ions at a ph = 7? mol/l what is the concentration of oh– ions at a ph = 7? mol/l what is the ratio of h+ ions to oh– ions at a ph = 7? : 1
Answers: 1

Chemistry, 22.06.2019 08:00
Identify a strong intermolecular force of attraction between an alcohol
Answers: 1

You know the right answer?
Calculate the standard cell potential at 25 ∘c for the reaction x(s)+2y+(aq)→x2+(aq)+2y(s) where δh∘...
Questions

History, 12.11.2020 21:20


Mathematics, 12.11.2020 21:20



Social Studies, 12.11.2020 21:20


Mathematics, 12.11.2020 21:20

Biology, 12.11.2020 21:20


Chemistry, 12.11.2020 21:20



Mathematics, 12.11.2020 21:20

History, 12.11.2020 21:20

Chemistry, 12.11.2020 21:20

Physics, 12.11.2020 21:20

Mathematics, 12.11.2020 21:20


Mathematics, 12.11.2020 21:20