
Chemistry, 03.01.2020 00:31 joneswilliam141236
Asample of caco3 (molar mass 100. g) was reported as being 30. percent ca. assuming no calcium was present in any impurities, calculate the percent of caco3 in the sample.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 18:30
Asample of the male sex hormone testosterone, c19h28o2, contains 3.88×10^21 atoms of hydrogen.(a) how many atoms of carbon does it contain? (b) how many molecules of testosterone does it contain? (c) how many moles of testosterone does it contain? (d) what is the mass of this sample in grams?
Answers: 1

Chemistry, 22.06.2019 11:20
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1

Chemistry, 22.06.2019 15:00
‘which reaction would most likely require the use of an inert electrode?
Answers: 1

Chemistry, 22.06.2019 20:00
How are the terms group and period used on the periodic table
Answers: 1
You know the right answer?
Asample of caco3 (molar mass 100. g) was reported as being 30. percent ca. assuming no calcium was p...
Questions


English, 09.03.2021 22:50



Health, 09.03.2021 22:50


Mathematics, 09.03.2021 22:50



Mathematics, 09.03.2021 22:50

Mathematics, 09.03.2021 22:50

World Languages, 09.03.2021 22:50

Advanced Placement (AP), 09.03.2021 22:50

Mathematics, 09.03.2021 22:50

Mathematics, 09.03.2021 22:50




Geography, 09.03.2021 22:50

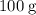 .The mass of one mole of Ca atoms is (numerically) the same as the relative atomic mass of this element:
.The mass of one mole of Ca atoms is (numerically) the same as the relative atomic mass of this element: 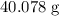 .
.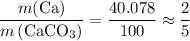 .
.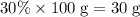 of Ca atoms. Assuming that the impurity does not contain any Ca. In other words, all these Ca atoms belong to CaCO₃. Apply the ratio
of Ca atoms. Assuming that the impurity does not contain any Ca. In other words, all these Ca atoms belong to CaCO₃. Apply the ratio 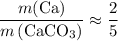 :
: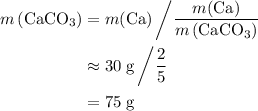 .
.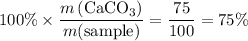 .
.

