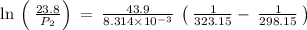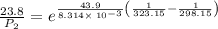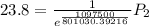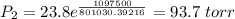Chemistry, 03.01.2020 02:31 jeffhuffle17
The vapor pressure of water at 25 degrees celsius is 23.8 torr, and the heat of vaporization of water at 25 degrees celsius is 43.9 kj/mol. calculate the vapor pressure of water at 50 degrees celsius.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 15:30
Zinc + lead(ii) nitrate yield zinc nitrate + leadwhat's the chemical equation for this?
Answers: 1

Chemistry, 22.06.2019 06:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 12:30
Consider the four elements above. which one of these elements will combine with oxygen in a 1: 1 ratio?
Answers: 3
You know the right answer?
The vapor pressure of water at 25 degrees celsius is 23.8 torr, and the heat of vaporization of wate...
Questions

Geography, 15.04.2020 22:43

History, 15.04.2020 22:43


History, 15.04.2020 22:43

History, 15.04.2020 22:43

Spanish, 15.04.2020 22:43

Biology, 15.04.2020 22:43

History, 15.04.2020 22:43



History, 15.04.2020 22:43



Mathematics, 15.04.2020 22:43



Mathematics, 15.04.2020 22:43

Physics, 15.04.2020 22:43

Computers and Technology, 15.04.2020 22:43

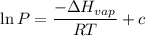
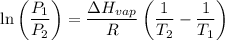
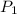 = 23.8 torr
= 23.8 torr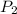 = ?
= ?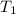 = 25°C
= 25°C
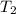 = 50 °C
= 50 °C