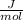Chemistry, 03.01.2020 03:31 lucywood2024
Carbon monoxide at 25°c and steam at 150°c are fed to a continuous water-gas shift reactor. the product gas, which contains 50.0 mole% h2, 40.0% , and the balance emerges at 500°c at a rate of 2.50m3/h and goes to a condenser. the gas and liquid streams leaving the condenser are in equilibrium at 15°c and 1 bar. the liquid may be taken to be pure w"a) calculate the % excess steam fed to the reactor and rate of condensation of the water (kg/h) leaving the condensor. b) calculate the rate (kw) at which heat must be removed from the condensor. c) calculate the rate of heat transfer (kw) to or from the reactor (state which it is).

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 08:00
Define dew point. i am writing this part to be able to ask the question
Answers: 1

Chemistry, 22.06.2019 08:30
Which of the following would have less momentum than a 52 kg cheetah running at 10 m/s?
Answers: 2

Chemistry, 22.06.2019 10:50
A100 kmol/h stream that is 97 mole% carbon tetrachloride (ccl4) and 3% carbon disulfide (cs2) is to be recovered from the bottom of a distillation column. the feed to the column is 16 mole% cs2 and 84% ccl4, and 2% of the ccl4 entering the column is contained in the overhead stream leaving the top of the column. calculate the mass and mole fractions of ccl4 in the overhead stream, and determine the molar flow rates of ccl4 and cs2 in the overhead and feed streams. 12. mw_ccla- 153.82; mw_cs2-76.14.
Answers: 3

Chemistry, 22.06.2019 15:30
Which suspect most likely committed the robbery and how do you know
Answers: 2
You know the right answer?
Carbon monoxide at 25°c and steam at 150°c are fed to a continuous water-gas shift reactor. the prod...
Questions


Biology, 20.01.2021 08:50

Mathematics, 20.01.2021 08:50


Social Studies, 20.01.2021 08:50

Mathematics, 20.01.2021 08:50


Chemistry, 20.01.2021 08:50




Mathematics, 20.01.2021 08:50


Biology, 20.01.2021 08:50

Mathematics, 20.01.2021 08:50

Mathematics, 20.01.2021 08:50


Physics, 20.01.2021 08:50

Mathematics, 20.01.2021 08:50

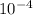 kW
kW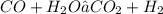
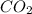 = 40 moles
= 40 moles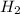 = 40 moles
= 40 moles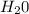 = 20 moles
= 20 moles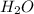
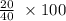 %
%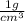
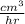
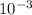
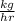
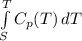
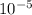 (-16324.231)
(-16324.231)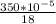 (-16324.231) *
(-16324.231) *