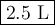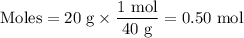Chemistry, 04.01.2020 01:31 MsShreve9939
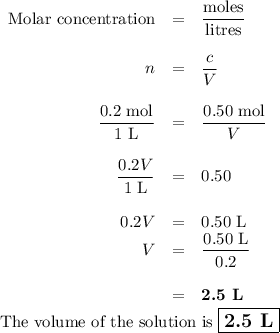
The molarity of a sodium hydroxide (naoh) solution is 0.2 m. the molar mass of naoh is 40 g/mol. if the solution contains 20 g of sodium hydroxide dissolved in water, what is the volume of the solution?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3

Chemistry, 22.06.2019 09:30
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1

Chemistry, 22.06.2019 10:00
Miner's coal distributors does not mine coal itself, nor does it even store or handle the coal. instead, miner's solicits orders for low sulfur coal from other firms, then purchases the required amount from suppliers and directs them to ship the coal to its customers. what is miner's
Answers: 1

Chemistry, 22.06.2019 11:50
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2
You know the right answer?
The molarity of a sodium hydroxide (naoh) solution is 0.2 m. the molar mass of naoh is 40 g/mol. if...
Questions


English, 05.05.2020 04:19



Computers and Technology, 05.05.2020 04:19


English, 05.05.2020 04:19

History, 05.05.2020 04:19

English, 05.05.2020 04:19



English, 05.05.2020 04:19


Mathematics, 05.05.2020 04:19




History, 05.05.2020 04:19

Biology, 05.05.2020 04:19

English, 05.05.2020 04:19