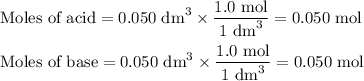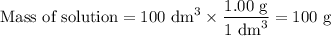Chemistry, 05.01.2020 17:31 gharrell03
50cm3 of 1 mol/dm3 hcl at 30°c was mixed with 50cm3 of 1mol/dm3 naoh at 30°c in a styrofoam calorimeter. the temperature of the calorimeter rose by 4.5°c. calculate the heat of reaction per mol of h20 formed.( heat capacity of the calorimeter is 50j/°c

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:00
Which set of characteristics best describes igneous rock? a) largest type of rock, made of organic matter, hardest type of rock b) least abundant type of rock, made of other rocks, made mostly of minerals c) found on all continents, contains wavy bands of stripes, contains fossils d) most abundant type in earth's crust, made of magma/lava, contains no fossils
Answers: 1

Chemistry, 22.06.2019 12:50
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1

Chemistry, 22.06.2019 19:00
How many moles of cu are needed to react with 5.8 moles of agno3? cu + 2 agno3 → cu(no3)2 + 2 ag
Answers: 3

Chemistry, 23.06.2019 01:00
You wish to prepare a buffer consisting of acetic acid and sodium acetate with a total acetic acetate plus acetate concentration of 250 mm and a ph of 5. what concentrations of acetic acid and sodium acetate should you use
Answers: 1
You know the right answer?
50cm3 of 1 mol/dm3 hcl at 30°c was mixed with 50cm3 of 1mol/dm3 naoh at 30°c in a styrofoam calorime...
Questions


Mathematics, 21.05.2021 05:20






Advanced Placement (AP), 21.05.2021 05:20



Advanced Placement (AP), 21.05.2021 05:20

Chemistry, 21.05.2021 05:20


Mathematics, 21.05.2021 05:20

Social Studies, 21.05.2021 05:20


Mathematics, 21.05.2021 05:20



Mathematics, 21.05.2021 05:20