ch4(g)+2o2-> co2(g)+2h2o(g) δh1=-802 kj

Chemistry, 06.01.2020 01:31 nikidastevens36
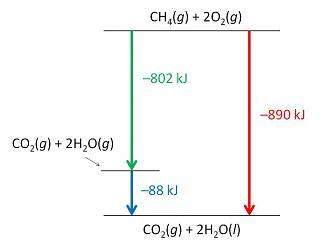
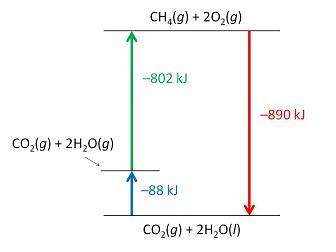
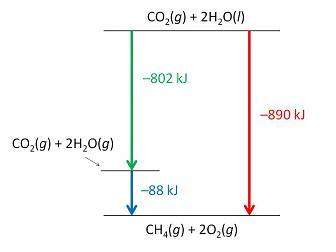
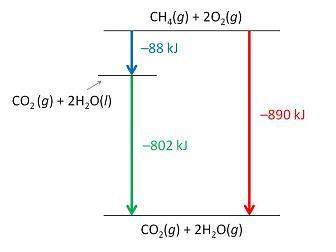
Consider the following intermediate reactions.
ch4(g)+2o2-> co2(g)+2h2o(g) δh1=-802 kj
2h2o(g)-> 2h2o(i) δh2=-88 kj
the overall chemical reaction is as follows.
ch4(g)+2o2(g)-> co2(g)+2h2o(i) δh2=-890 kj
what is the correct enthalpy diagram using the hess law for this system?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:00
In an investigation that uses the scientific method, which step immediately follows making a hypothesis? o summarizing the results o asking a question o making observations designing an experiment mark this and retum save and exit next submit
Answers: 2

Chemistry, 22.06.2019 12:30
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3

Chemistry, 22.06.2019 14:30
Is a pencil falling to the floor anon contact force, a force, or a contact force
Answers: 1

Chemistry, 22.06.2019 15:00
Many ionic compounds and a few highly polar covalent compounds are because they completely ionize in water to create a solution filled with charged ions that can conduct an electric current.
Answers: 1
You know the right answer?
Consider the following intermediate reactions.
ch4(g)+2o2-> co2(g)+2h2o(g) δh1=-802 kj
ch4(g)+2o2-> co2(g)+2h2o(g) δh1=-802 kj
Questions

History, 22.06.2019 18:00

Mathematics, 22.06.2019 18:00

Physics, 22.06.2019 18:00

History, 22.06.2019 18:00

Business, 22.06.2019 18:00

Health, 22.06.2019 18:00

Mathematics, 22.06.2019 18:00


Business, 22.06.2019 18:00

Mathematics, 22.06.2019 18:00

Mathematics, 22.06.2019 18:00

History, 22.06.2019 18:00



Mathematics, 22.06.2019 18:00

History, 22.06.2019 18:00

Mathematics, 22.06.2019 18:00

Biology, 22.06.2019 18:00

Mathematics, 22.06.2019 18:00



