
Chemistry, 06.01.2020 04:31 Silkyruthie
Consider the half reactions below for a chemical reaction.
> zn²⁺(aq)+2e⁻
cu²⁺(aq)+2e⁻> cu(s)
what is the overall equation for this chemical reaction?
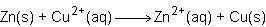

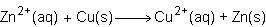
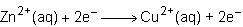

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:30
100 points answer quick the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 04:00
How do scientists think that gravity affected the formation of our solar system?
Answers: 1

Chemistry, 22.06.2019 12:00
What is the subscript for oxygen in its molecular formula
Answers: 1

Chemistry, 22.06.2019 18:30
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2
You know the right answer?
Consider the half reactions below for a chemical reaction.
> zn²⁺(aq)+2e⁻
cu²⁺(aq)+2e...
> zn²⁺(aq)+2e⁻
cu²⁺(aq)+2e...
Questions

Mathematics, 22.04.2021 18:10

Health, 22.04.2021 18:10

Arts, 22.04.2021 18:10

Computers and Technology, 22.04.2021 18:10

Mathematics, 22.04.2021 18:10




Mathematics, 22.04.2021 18:10


Business, 22.04.2021 18:10

Mathematics, 22.04.2021 18:10

Health, 22.04.2021 18:10

Mathematics, 22.04.2021 18:10




Mathematics, 22.04.2021 18:10


Biology, 22.04.2021 18:10



