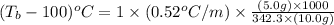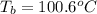Chemistry, 06.01.2020 18:31 shelbycade230
If 5.0 grams of sucrose, c12h22o11, are dissolved in 10.0 grams of water, what will be the boiling point of the resulting solution?

Answers: 3
Another question on Chemistry



Chemistry, 22.06.2019 15:00
Describe what happens to the molecules as water goes from ice to liquid to vapor. be sure to explain what happens to the temperature during the phase changes.
Answers: 2

Chemistry, 22.06.2019 16:30
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1
You know the right answer?
If 5.0 grams of sucrose, c12h22o11, are dissolved in 10.0 grams of water, what will be the boiling p...
Questions


Mathematics, 26.07.2019 10:30

Mathematics, 26.07.2019 10:30





Biology, 26.07.2019 10:30

History, 26.07.2019 10:30

History, 26.07.2019 10:30





History, 26.07.2019 10:30

Advanced Placement (AP), 26.07.2019 10:30


Mathematics, 26.07.2019 10:30


Advanced Placement (AP), 26.07.2019 10:30

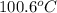
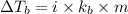
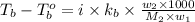
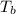 = boiling point of solution = ?
= boiling point of solution = ?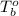 = boiling point of water =
= boiling point of water = 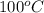
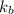 = boiling point constant =
= boiling point constant = 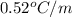
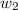 = mass of solute (sucrose) = 5.0 g
= mass of solute (sucrose) = 5.0 g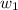 = mass of solvent (water) = 10.0 g
= mass of solvent (water) = 10.0 g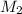 = molar mass of solute (sucrose) = 342.3 g/mol
= molar mass of solute (sucrose) = 342.3 g/mol