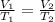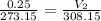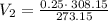Chemistry, 06.01.2020 18:31 allycoops666666
Acylinder with a movable piston contains a fixed amount of gas at a constant pressure. initially, the cylinder contains 0.25 liters of air at 0 degrees celsius. when the temperature is increased to 35 degrees celsius, the air will occupy what volume?
a. 0.28 l
b. 0.88 l
c. 8.75 l
d. 35.25 l

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:00
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2


Chemistry, 22.06.2019 17:30
Observation and experimentation have led many scientists to accept a theory about the origin of the universe. this theory is called the big bang theory. scientific evidence collected and observed by scientists around the world suggests that the universe is ever expanding from a hot and dense initial state. what makes this a scientific theory? (2 points)
Answers: 2

Chemistry, 22.06.2019 19:10
Δu of , in kj/kg, as it isto k, (a)as a of , (b) at , (c) at .
Answers: 2
You know the right answer?
Acylinder with a movable piston contains a fixed amount of gas at a constant pressure. initially, th...
Questions

Mathematics, 17.07.2020 02:01




Mathematics, 17.07.2020 02:01

History, 17.07.2020 02:01

English, 17.07.2020 02:01


Mathematics, 17.07.2020 02:01


Mathematics, 17.07.2020 02:01

Arts, 17.07.2020 02:01


Mathematics, 17.07.2020 02:01



Mathematics, 17.07.2020 02:01

Mathematics, 17.07.2020 02:01