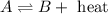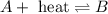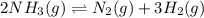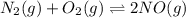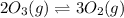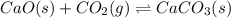Chemistry, 06.01.2020 19:31 karsynraine9419
How will an increase in temperature affect each of the following equilibria? how will a decrease in the volume of the reaction vessel affect each?
(a) 2nh3(g) ⇌ n2(g)+3h2(g) δh = 92kj
(b) n2(g) + o2(g) ⇌ 2no(g) δh =181kj
(c) 2o3(g) ⇌ 3o2(g) δh = − 285kj
(d) cao(s) + co2(g) ⇌ caco3(s) δh = − 176kj

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:40
In an effort to address concerns about global warming, a power plant in portland,oregon is designed to take all of its exhaust gases from its boilers and recycle the co2 using the solvay process to make sodium hydrogen carbonate. the reaction is shown below. nh3(g) + h2o(l) + co2(g) + nacl(aq) → nahco3(aq) + nh4cl(aq) how many liters each of nh3 and co2 (both at stp) would be consumed to produce 3.00 kg of sodium bicarbonate? the volume of both nh3 and co2 would be
Answers: 1

Chemistry, 22.06.2019 12:30
Write the chemical formula for a compound that is made of an element from group 1 and an element from group 17
Answers: 1

Chemistry, 23.06.2019 04:40
Equal numbers of moles of he(g), ar(g), and ne(g) are placed in a glass vessel at room temperature. if the vessel has a pinhole-sized leak, which of the following will be true regarding the relative values of the partial pressures of the gases remaining in the vessel after some of the gas mixture has effused?
Answers: 1

You know the right answer?
How will an increase in temperature affect each of the following equilibria? how will a decrease in...
Questions



Mathematics, 04.07.2019 17:00

Mathematics, 04.07.2019 17:00

History, 04.07.2019 17:00


Biology, 04.07.2019 17:00

Biology, 04.07.2019 17:00

Mathematics, 04.07.2019 17:00

Mathematics, 04.07.2019 17:00

Mathematics, 04.07.2019 17:00


Health, 04.07.2019 17:00




English, 04.07.2019 17:00

Chemistry, 04.07.2019 17:00

English, 04.07.2019 17:00