
Chemistry, 06.01.2020 21:31 Mattixwillard
Write the molecular and net ionic equations for the reaction in aqueous solution of sulfuric acid with excess lithium hydroxide.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 13:00
When light strikes a plane mirror, images form in locations where light does not actually reach. it only appears to the observer as though the light were coming from this position. what type of image is formed?
Answers: 2

Chemistry, 21.06.2019 20:00
Drag each number to the correct location on the equation. each number can be used more than once, but not all numbers will be used. balance the equation with the coefficients. 2 3 4 5 kclo3 -> kcl + o2
Answers: 1

Chemistry, 21.06.2019 20:30
In a laboratory experiment, a fermenting aqueous solution of glucose and yeast produces carbon dioxide gas and ethanol. the solution was heated by burning natural gas in a bunsen burner to distill the ethanol that formed in the flask. during the distillation, the ethanol evaporated and then condensed in the receiving flask. the flame of the burner was kept too close to the bottom of the flask and some of the glucose decomposed into a black carbon deposit on the inside of the flask. during this experiment the following changes occurred. which of these changes involved a physical change and not a chemical change? check all that apply. 1-condensation of ethanol 2-evaporation of ethanol 3- formation of carbon dioxide gas from glucose burning of natural gas 4-formation of ethanol from glucose by yeast 5-formation of a carbon deposit inside the flask
Answers: 2

You know the right answer?
Write the molecular and net ionic equations for the reaction in aqueous solution of sulfuric acid wi...
Questions


Mathematics, 08.12.2020 22:10


Business, 08.12.2020 22:10

Biology, 08.12.2020 22:10

Mathematics, 08.12.2020 22:10




Physics, 08.12.2020 22:10

English, 08.12.2020 22:10

Mathematics, 08.12.2020 22:10


Mathematics, 08.12.2020 22:10


Mathematics, 08.12.2020 22:10

Chemistry, 08.12.2020 22:10


Mathematics, 08.12.2020 22:10

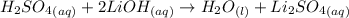
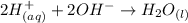
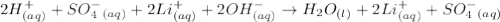
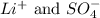 are the spectator ions.
are the spectator ions.


