Chemistry, 08.01.2020 09:31 lisa123465
1. how many moles of nitrogen monoxide can be made using 5.0 moles of oxygen in
the following composition reaction?
n2 + o2 -> no
2. the neutralization of an acid with a base is a double replacement reaction in which a salt and water are formed. if you start with 25 moles of hcl and neutralize it with
naoh how many moles of nacl will be formed?
hcl + naoh->
3. a car burns gasoline (octane – c8h18) with oxygen. if you drive to salt lake and
burn 150 moles of octane how many moles of carbon dioxide are you producing?
4. if 25 gram of magnesium combines with oxygen in a composition reaction, how
many moles of magnesium oxide will be formed?
mg + o2 -> mgo
5. lithium reacts with water in a single replacement reaction. how many moles of
hydrogen gas a produced by 10 grams of lithium?
li + hoh ->
6. barium chloride reacts with sodium sulfate in a double replacement reaction. how
many grams of barium chloride are required to react with 5 moles of sodium sulfate?
7. magnesium carbonate when heated decomposes to form magnesium oxide and carbon dioxide. how many grams of magnesium oxide will be formed if 20 grams of
magnesium carbonate are heated?
mgco3 -> mgo + co2
8. if 70 grams of gold iii chloride decomposes into its elements, how many grams of
gold will be produced?
aucl3 -->
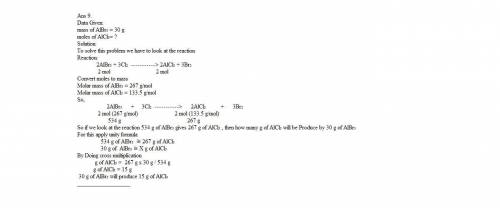
9. chlorine is more reactive element than bromine, thus chlorine will replace bromine in compound through a single replacement reaction. if 30 grams of aluminum bromide react with chlorine in this fashion how many grams of aluminum chloride will be formed?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:30
The density of an unknown gas at 98°c and 740 mmhg is 2.50 g/l. what is the molar mass of the gas with work showed?
Answers: 1

Chemistry, 22.06.2019 04:30
How much energy is made when a pice of wood burns. how do you know
Answers: 2

Chemistry, 22.06.2019 04:30
Why are people not able to scuba dive in the deep part of the ocean
Answers: 2

Chemistry, 22.06.2019 12:30
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2
You know the right answer?
1. how many moles of nitrogen monoxide can be made using 5.0 moles of oxygen in
the following...
the following...
Questions

Mathematics, 18.03.2021 02:40


Social Studies, 18.03.2021 02:40





Chemistry, 18.03.2021 02:40

Mathematics, 18.03.2021 02:40


Mathematics, 18.03.2021 02:40



Mathematics, 18.03.2021 02:40

Mathematics, 18.03.2021 02:40


Mathematics, 18.03.2021 02:40

Mathematics, 18.03.2021 02:40