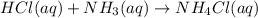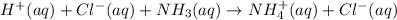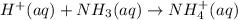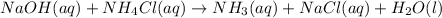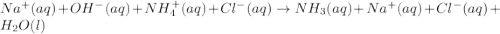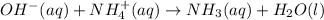Chemistry, 08.01.2020 18:31 addisonrausch
Write a net ionic equation for the reaction of hydrochloric acid with ammonia in aqueous solution. and write a net ionic equation for the reaction sodium hydroxide (aq) + ammonium chloride (aq).

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 17:00
Initially, the balloon had 3.0 liters of gas at a pressure of 400 kpa and was at a temperature of 294 k. if the balloon is cooled to 277 k and its volume decreased to 1 l, what will the new pressure in the balloon be?
Answers: 1

Chemistry, 21.06.2019 21:00
Match the following items. 1. high-intensity bundle of energy being emitted from some decaying nuclei gamma ray 2. particle radiating from the nucleus of some atoms beta particle 3. negative particle identical to an electron but radiating from a decaying nucleus alpha particle
Answers: 1


Chemistry, 22.06.2019 06:40
Which statement correctly describes metallic bonds? a. they form when certain atoms lose electrons and other atoms gain electrons. b. they involve an attraction between anions and cations. they always involvpoth a metal and a nonmetal. d. they can only form between atoms of the same element. e. they form because electrons can move freely between atoms.
Answers: 3
You know the right answer?
Write a net ionic equation for the reaction of hydrochloric acid with ammonia in aqueous solution. a...
Questions

Biology, 05.10.2019 21:10

Mathematics, 05.10.2019 21:10

History, 05.10.2019 21:10

Chemistry, 05.10.2019 21:10

Chemistry, 05.10.2019 21:10

History, 05.10.2019 21:10


Mathematics, 05.10.2019 21:10

Business, 05.10.2019 21:10

Geography, 05.10.2019 21:10

History, 05.10.2019 21:10




Computers and Technology, 05.10.2019 21:10

History, 05.10.2019 21:10



Mathematics, 05.10.2019 21:10