
Chemistry, 09.01.2020 01:31 zachspencer6444
Given the following information, what is the concentration of h2o(g) at equilibrium? [h2s](eq) = 0.671 m [o2](eq) = 0.587 m kc = 1.35 2h2s(g) + o2(g) ⇌ 2s(s) + 2h2o(g)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:30
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2

Chemistry, 22.06.2019 10:10
When water dissociates, each water molecule splits into a hydroxide ion and a) h 3 o + b) a hydrogen atom c) a hydrogen ion d) h 2 o e) oh —
Answers: 2

Chemistry, 22.06.2019 11:30
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. you'll find ksp data in the aleks data tab. compounds does solubility change with ph
Answers: 3

Chemistry, 22.06.2019 16:40
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3
You know the right answer?
Given the following information, what is the concentration of h2o(g) at equilibrium? [h2s](eq) = 0....
Questions

Mathematics, 12.07.2019 14:00

Mathematics, 12.07.2019 14:00


Mathematics, 12.07.2019 14:00







Mathematics, 12.07.2019 14:00


English, 12.07.2019 14:00

English, 12.07.2019 14:00

English, 12.07.2019 14:00




Mathematics, 12.07.2019 14:00

Mathematics, 12.07.2019 14:00

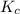
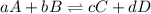
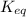 is written as:
is written as:![K_{c}=\frac{[C]^c[D]^d}{[A]^a[B]^b}](/tpl/images/0447/6972/b6f47.png)
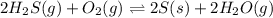
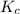 for above equation is:
for above equation is:![K_c=\frac{[H_2O]^2}{[H_2S]^2\times [O_2]}](/tpl/images/0447/6972/73c62.png)
![[H_2S]_{eq}=0.671M](/tpl/images/0447/6972/623cf.png)
![[O_2]_{eq}=0.587M](/tpl/images/0447/6972/4ff7f.png)

![1.35=\frac{[H_2O]^2}{(0.671)^2\times 0.587}](/tpl/images/0447/6972/14791.png)
![[H_2O]=\sqrt{(1.35\times 0.671\times 0.671\times 0.587)}=0.597M](/tpl/images/0447/6972/73db2.png)


