
Chemistry, 09.01.2020 01:31 chloedonyes
For the reaction, 2 n2o5--> 4 no2+o2, the rate of formation of no2 is 0.004 mol^-1 s^-1.a) calculate the rate of disappearance of n2o5.b) calculate the rate of appearance of o2.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 17:30
Given that the molar mass of nano3 is 85.00 g/mol, what mass of nano3 is needed to make 4.50 l of a 1.50 m nano3solution? use .6.75 g18.9 g255 g574 g
Answers: 1

Chemistry, 21.06.2019 22:00
Which of the following statements is true about planck’s law
Answers: 1

Chemistry, 22.06.2019 11:00
Which statement correctly identifies the scientific question and describes why the question is scientific? question 1 refers to the supernatural.question 2 reflects a moral or social value.question 3 refers to something that can be measured.question 4 reflects a question that can’t be observed.
Answers: 1

Chemistry, 22.06.2019 12:00
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1
You know the right answer?
For the reaction, 2 n2o5--> 4 no2+o2, the rate of formation of no2 is 0.004 mol^-1 s^-1.a) calcu...
Questions

Mathematics, 02.07.2019 14:30

Spanish, 02.07.2019 14:30


Mathematics, 02.07.2019 14:30


Mathematics, 02.07.2019 14:30


Mathematics, 02.07.2019 14:30



History, 02.07.2019 14:30

Mathematics, 02.07.2019 14:30


History, 02.07.2019 14:30

Chemistry, 02.07.2019 14:30

Geography, 02.07.2019 14:30

English, 02.07.2019 14:30

Mathematics, 02.07.2019 14:30


English, 02.07.2019 14:30

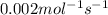
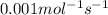
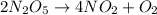
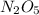 =
= ![-\frac{1d[N_2O_5]}{2dt}](/tpl/images/0447/6971/30fba.png)
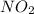 =
= ![\frac{1d[NO_2]}{4dt}](/tpl/images/0447/6971/b576c.png)
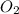 =
= ![\frac{1d[O_2]}{dt}](/tpl/images/0447/6971/6b167.png)
![NO_2=+\frac{1d[NO_2]}{dt}=0.004mol^{-1}s^{-1}](/tpl/images/0447/6971/9cf34.png)
![N_2O_5=\frac{2}{4}\times \frac{1d[NO_2]}{dt}=\frac{2}{4}\times 0.004=0.002mol^{-1}s^{-1}](/tpl/images/0447/6971/202ca.png)
![\frac{1}{4}\times \frac{1d[NO_2]}{dt}=\frac{1}{4}\times 0.004=0.001mol^{-1}s^{-1}](/tpl/images/0447/6971/a8812.png)


