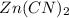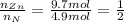Chemistry, 09.01.2020 03:31 twalters88
An unknown compound has the following chemical formula: zn(cn)x where x stands for a whole number. measurements also show that a certain sample of the unknown compound contains 9.7 mol of nitrogen and 4.9 mol of zinc. write the complete chemical formula for the unknown compound.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:30
Calculate the expected ph values of the buffer systems from the experiments (a,b,c,d), using the henderson- hasselbalch equation, ph-pka+log[a-]/[ha]. use for pka values carbonic acid= 6.37, and acetic acid= 4.75.
Answers: 2

Chemistry, 22.06.2019 00:30
The clouds are grey and ground is wet. a quantitative b qualitative
Answers: 1


Chemistry, 22.06.2019 09:40
How many grams of aluminum will there be in 98g of al2o3?
Answers: 1
You know the right answer?
An unknown compound has the following chemical formula: zn(cn)x where x stands for a whole number....
Questions

Mathematics, 19.05.2021 22:10


English, 19.05.2021 22:10








Mathematics, 19.05.2021 22:10



Mathematics, 19.05.2021 22:10




Mathematics, 19.05.2021 22:10


History, 19.05.2021 22:10