
Chemistry, 09.01.2020 06:31 JocelynC24
Ethanol melts at -114 degree c. the enthalpy of fusion
is5.02kj/mol. the specific heat of solid and liquid ethanol are
0.97jk/g-k and 2.31 j/g-k, respectively. how much heat(kj) is
needed toconvert 25.0 g of solid ethanol at -135 degree c to liquid
ethanolat -50 degree c?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:30
When svante arrhenius first proposed his acid-base theory, he was a doctoral candidate. his professors thought his ideas were unfounded. within a decade, the arrhenius theory of acid-base was widely accepted and praised within the scientific world. arrhenius defined acids as compounds having ionizable hydrogen and bases as compounds with ionizable a) barium. b) hydronium. c) hydroxide. d) oxygen.
Answers: 3

Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 1

Chemistry, 22.06.2019 20:20
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1

Chemistry, 22.06.2019 22:30
What is the value of the standard enthalpy of formation of an element in its most stable form?
Answers: 3
You know the right answer?
Ethanol melts at -114 degree c. the enthalpy of fusion
is5.02kj/mol. the specific heat of soli...
is5.02kj/mol. the specific heat of soli...
Questions



Mathematics, 28.06.2019 11:30

Mathematics, 28.06.2019 11:30


English, 28.06.2019 11:30


History, 28.06.2019 11:30


Mathematics, 28.06.2019 11:30

History, 28.06.2019 11:30

History, 28.06.2019 11:30

Mathematics, 28.06.2019 11:30





Biology, 28.06.2019 11:30


Business, 28.06.2019 11:30

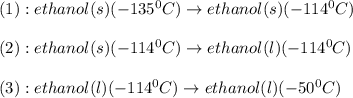
![\Delta H=[m\times c_{p,s}\times (T_{final}-T_{initial})]+n\times \Delta H_{fusion}+[m\times c_{p,l}\times (T_{final}-T_{initial})]+n\times \Delta H_{vap}+[m\times c_{p,g}\times (T_{final}-T_{initial})]](/tpl/images/0448/1498/e4ef0.png)
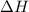 = enthalpy change = ?
= enthalpy change = ?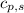 = specific heat of solid ethanol= 0.97 J/gK
= specific heat of solid ethanol= 0.97 J/gK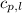 = specific heat of liquid ethanol = 2.31 J/gK
= specific heat of liquid ethanol = 2.31 J/gK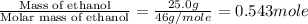
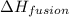 = enthalpy change for fusion = 5.02 KJ/mole = 5020 J/mole
= enthalpy change for fusion = 5.02 KJ/mole = 5020 J/mole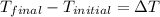 = change in temperature
= change in temperature ![\Delta H=[25.0 g\times 0.97J/gK\times (-114-(-135)K]+0.534mole\times 5020J/mole+[25.0g\times 2.31J/gK\times (-50-(-114))K]](/tpl/images/0448/1498/b9d5c.png)
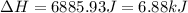 (1 KJ = 1000 J)
(1 KJ = 1000 J)


