
Chemistry, 09.01.2020 06:31 jessixa897192
The distribution constant for a molecule x between n-hexane and water
is 13.5. calculate the percent of x remaining in the water phase that
was originally 0.0100 m in z after extraction of 50.0 ml of water with
4 extractions of 10.0 ml portions of n-hexane.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:00
Choose all the answers that apply. ionic compounds dissolve easily in water do not dissolve in water have low melting points have high melting points conduct electricity when melted
Answers: 1

Chemistry, 22.06.2019 06:10
56.16 gregor mendel was the first scientist to use statistics to analyze scientific data. before mendel's experiments, scientists believed that organisms acquired traits from their environment and passed them on to their offspring. after mendel's discoveries were accepted, scientists realized that traits passed to offspring were the result of genes being passed from parents to offspring. this is an example of pls
Answers: 1

Chemistry, 22.06.2019 11:30
Aperfume bottle is dropped in the corner of a room. the odor of the perfume can be detected on the other side of the room. which statement best describes this observation?
Answers: 2

You know the right answer?
The distribution constant for a molecule x between n-hexane and water
is 13.5. calculate the p...
is 13.5. calculate the p...
Questions

Computers and Technology, 11.10.2020 23:01

Mathematics, 11.10.2020 23:01



History, 11.10.2020 23:01

Geography, 11.10.2020 23:01




English, 11.10.2020 23:01

English, 11.10.2020 23:01


Mathematics, 11.10.2020 23:01

Biology, 11.10.2020 23:01


Mathematics, 11.10.2020 23:01

Health, 12.10.2020 01:01


Arts, 12.10.2020 01:01

Biology, 12.10.2020 01:01

![[X]_{i} = (\frac{V_{aq}}{V_{or}KD + V_{aq}})^{i} \cdot [X_{0}]](/tpl/images/0448/1496/650eb.png)
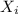 : is the concentration of A remaining in aqueous solution
: is the concentration of A remaining in aqueous solution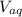 : is the volume of water
: is the volume of water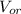 : is the volume of the organic solvent
: is the volume of the organic solvent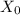 : is the original concentration of X
: is the original concentration of X![[X]_{i} = (\frac{50 mL}{10 mL \cdot 13.5 + 50 mL})^{4} \cdot 0.01 M = 5.34 \cdot 10^{-5} M](/tpl/images/0448/1496/4cebe.png)
![[X]_{i} = 5.34 \cdot 10^{-5} \cdot 100 \% = 0.005 \%](/tpl/images/0448/1496/48d0a.png)


