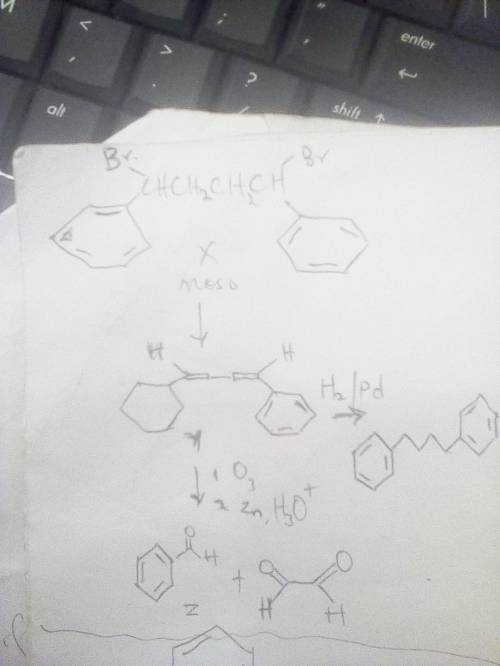
Compound x is optically inactive and has the
formulac16h16br2. on treatment
withstrong b...

Chemistry, 09.01.2020 06:31 kelseiroll8554
Compound x is optically inactive and has the
formulac16h16br2. on treatment
withstrong base, x gives hydrocarbon y,
c16h14.compound y absorbs 2 equivalents of
hydrogen when reduced over apalladium catalyst and reacts with
ozone to give two fragments. onefragment, z, is an aldehyde with
formulac7h6o. the other fragment is
glyoxal,(cho)2. write the reactions involved, and
suggeststructures for x, y, and z. what is the stereochemistry of
x?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:00
Match the mixture with the substance// i really need on this guys (it’s a pic btw)
Answers: 1

Chemistry, 22.06.2019 10:00
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 10:50
Determine the empirical formula for succinic acid that is composed of 40.60% carbon, 5.18% hydrogen, and 54.22% oxygen.
Answers: 1

Chemistry, 22.06.2019 12:30
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2
You know the right answer?
Questions


Mathematics, 25.09.2021 14:00


Mathematics, 25.09.2021 14:00


History, 25.09.2021 14:00


English, 25.09.2021 14:00

Physics, 25.09.2021 14:00

Mathematics, 25.09.2021 14:00

Social Studies, 25.09.2021 14:00

English, 25.09.2021 14:00

Mathematics, 25.09.2021 14:00

Mathematics, 25.09.2021 14:00


English, 25.09.2021 14:00


Mathematics, 25.09.2021 14:00