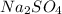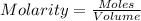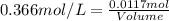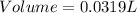Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 18:10
Using complete sentences, explain how to predict the products and balance the reaction between sulfuric acid and potassium hydroxide.
Answers: 1

Chemistry, 21.06.2019 21:30
Which substances have the lowest melting points: ionic covalent, or metallic
Answers: 1

Chemistry, 22.06.2019 07:30
In a reaction (at equilibrium) that makes more moles of gas than it consumes, what is the effect of increasing the pressure?
Answers: 1

Chemistry, 22.06.2019 14:50
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of tthe table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3
You know the right answer?
A5.05% (by mass) of aqueaus solution of sodium sulfate
isallowed to react with an excess of ba...
isallowed to react with an excess of ba...
Questions

Computers and Technology, 20.09.2019 12:20

English, 20.09.2019 12:20


Mathematics, 20.09.2019 12:20


English, 20.09.2019 12:20

Mathematics, 20.09.2019 12:20

Computers and Technology, 20.09.2019 12:20


Health, 20.09.2019 12:20

Mathematics, 20.09.2019 12:20

Biology, 20.09.2019 12:20



Biology, 20.09.2019 12:20


Health, 20.09.2019 12:20



English, 20.09.2019 12:20

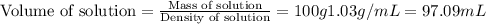
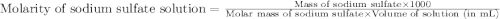
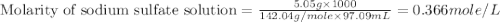
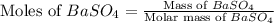
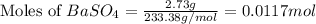
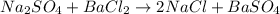
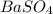 produced from 1 mole of
produced from 1 mole of