
Chemistry, 09.01.2020 07:31 dareaalcaam111
The rate law for the decomposition of aqueous hydrogenperoxide
(h2o2) at 70 degrees celcius is first order in
with k=.0347 min to the negative 1.
a) calculate the half-life (t1/2) for this reaction at
70degrees c
b) given that the initial concentration of h202 is
.300mcalculate the concentration of h2o2 after 60 minutes
haselapsed.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:00
Which of the following methods uses the decay of atomic particles in an object to find its exact age? a. fossil dating b. geologic dating c. radioactive dating d. relative dating
Answers: 1

Chemistry, 22.06.2019 11:40
Enzymes affect the reactions in living cells by changing the
Answers: 3

Chemistry, 23.06.2019 00:00
Mercury turns to a vapor at 629.88 k. how much heat is lost when 75.0 g of mercury vapor at 650 k condenses to a liquid at 297 k?
Answers: 1

Chemistry, 23.06.2019 07:00
The image compares the arrangement of electrons in two different neutral atoms. a figure labeled atom q has a shaded sphere at the center of three concentric circles. the innermost circle has two black spheres. the middle circle has six black spheres. to the left of this figure is another figure labeled atom p. atom p has a shaded sphere at the center of three concentric circles. the innermost circle has two black spheres. the middle circle has seven black spheres. which of the following best explains the position of the two atoms in the periodic table?
Answers: 2
You know the right answer?
The rate law for the decomposition of aqueous hydrogenperoxide
(h2o2) at 70 degrees celcius is...
(h2o2) at 70 degrees celcius is...
Questions








Social Studies, 26.07.2019 21:00







History, 26.07.2019 21:00



Mathematics, 26.07.2019 21:00

Biology, 26.07.2019 21:00

History, 26.07.2019 21:00

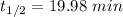
![[A_t]=0.037\ M](/tpl/images/0448/2196/db3b8.png)
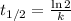
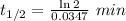
![[A_t]=[A_0]e^{-kt}](/tpl/images/0448/2196/1ef89.png)
![[A_t]](/tpl/images/0448/2196/5262c.png) is the concentration at time t
is the concentration at time t
![[A_0]](/tpl/images/0448/2196/9a686.png) is the initial concentration
is the initial concentration
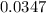 min⁻¹
min⁻¹
![[A_t]=0.300\times e^{-0.0347\times 60}\ M](/tpl/images/0448/2196/8ceed.png)
![[A_t]=0.3\times \frac{1}{e^{2.082}}\ M](/tpl/images/0448/2196/5c14c.png)


