
Chemistry, 10.01.2020 06:31 aidanwindsor1738
Asource of zinc metal can be zinc ore containing zinc(ii) sulfide. the ore is roasted in pure oxygen to produce the oxide and then reduced with carbon to form elemental zinc and carbon monoxide. 2 zns + o2 2 zno + 2 so2 zno + c zn + co a crucible containing a sample of 0.50 mol zns was roasted in pure oxygen, then reduced with 1.00 mol carbon. what mass remained in the crucible after cooling?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 21:30
Harry lives in a city, and he has a lung condition known as asthma. on certain days, harry has to stay inside because pollutants in the air make it difficult for him to breathe. which of these pollution sources are nonpoint sources that might bother harry if he goes outside? choose the two that apply.
Answers: 3

Chemistry, 23.06.2019 01:00
Which statement best describes isomers? a. isomers are alcohols that have the same functional group. b. isomers have at least one carbon-carbon double bond. c. isomers have the same molecular formula but different structural properties.
Answers: 1

Chemistry, 23.06.2019 02:00
Why does ammonia, nh3, behave as a base when it reacts with an acid? z
Answers: 2

Chemistry, 23.06.2019 10:20
Based on the equation, how many grams of br2 are required to react completely with 29.2 grams of alcl3? alcl3 + br2 → albr3 + cl2 48.7 grams 52.6 grams 56.7 grams 61.3 grams
Answers: 3
You know the right answer?
Asource of zinc metal can be zinc ore containing zinc(ii) sulfide. the ore is roasted in pure oxygen...
Questions

Social Studies, 01.01.2020 00:31

Social Studies, 01.01.2020 00:31


Biology, 01.01.2020 00:31




Physics, 01.01.2020 00:31


Social Studies, 01.01.2020 00:31

History, 01.01.2020 00:31

World Languages, 01.01.2020 00:31

Mathematics, 01.01.2020 00:31



English, 01.01.2020 00:31


Spanish, 01.01.2020 00:31

History, 01.01.2020 00:31

 ..[1]
..[1]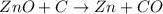 ..[2]
..[2] ..[3]
..[3]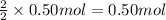 of Zn.
of Zn.

