
Chemistry, 11.01.2020 02:31 brenda0014
Propane burns in oxygen to produce carbon dioxide and water what is the percent yeild

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:10
Describe the difference between a. a hypothesis and a theory and b. an observation and an experiment.
Answers: 1

Chemistry, 22.06.2019 12:00
Ageochemist examines a piece of metal that he found in the soil. he performs tests to identify the metal from its density, electrical conductivity, and melting point. which statement best describes his investigation? a. he is determining physical properties that are sufficient to identify the metal.b. he is determining chemical properties that are sufficient to identify the metal.c. he is determining physical properties that are insufficient to identify the metal.d. he is determining chemical properties that are insufficient to identify the metal.
Answers: 3

Chemistry, 22.06.2019 16:00
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3

Chemistry, 22.06.2019 17:00
In a heat engine of 1000 j of heat enters the system and the piston does 500 j of work what is the final internal energy of the system if the inital energy was 2000 j we have to do all of these down here 1)write the equation 2)list out your know variables 3)plug the numbers into the equations 4)solve 5)write your solution statemtn that includes inital energuy and final energuy added
Answers: 1
You know the right answer?
Propane burns in oxygen to produce carbon dioxide and water what is the percent yeild...
Questions

Mathematics, 25.02.2021 14:00

Physics, 25.02.2021 14:00

Mathematics, 25.02.2021 14:00

Mathematics, 25.02.2021 14:00


Mathematics, 25.02.2021 14:00

Mathematics, 25.02.2021 14:00

English, 25.02.2021 14:00

Mathematics, 25.02.2021 14:00

Mathematics, 25.02.2021 14:00

Health, 25.02.2021 14:00


Arts, 25.02.2021 14:00

Mathematics, 25.02.2021 14:00


Computers and Technology, 25.02.2021 14:00

Geography, 25.02.2021 14:00


Mathematics, 25.02.2021 14:00

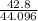 g.mol-1
g.mol-1
 44.0 g/mol
44.0 g/mol


