
Chemistry, 11.01.2020 04:31 isabella4141
Under the same conditions of temperature and pressure, which of the following gases would behave most like an ideal gas? ne, n₂, or ch₄? explain.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 22.06.2019 17:00
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2

Chemistry, 22.06.2019 18:00
Which statement best describes the he properties of iconic compounds ?
Answers: 1

Chemistry, 22.06.2019 23:30
The sum of the oxidation numbers in a neutral compound is always
Answers: 2
You know the right answer?
Under the same conditions of temperature and pressure, which of the following gases would behave mos...
Questions



History, 03.02.2020 06:03

Chemistry, 03.02.2020 06:03


Mathematics, 03.02.2020 06:03

Health, 03.02.2020 06:03

History, 03.02.2020 06:03



Spanish, 03.02.2020 06:03




English, 03.02.2020 06:03

Biology, 03.02.2020 06:03

Mathematics, 03.02.2020 06:03

Health, 03.02.2020 06:03


Mathematics, 03.02.2020 06:03

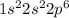
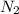 and
and 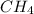 are reactive and hence, does not behave as ideal gas.
are reactive and hence, does not behave as ideal gas.

