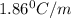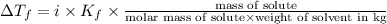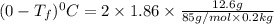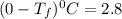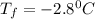Chemistry, 11.01.2020 07:31 tedrayoung1
Show the calculation of the freezing point of a solution made by dissolving 12.6 grams of the electrolyte nano3 in 200 grams of water. kf for water is 1.86 and the fp of pure water is 0oc.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:00
Which of the dna typing techniques do you think you would choose if you had to analyze a dna sample? why?
Answers: 1

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium
Answers: 1


Chemistry, 23.06.2019 01:30
Astudent states that 9.0 g of baking soda will form an unsaturated solution in 100 g of water. what do you need to know to decide whether this statement is correct? a. the temperature of the water and the molar mass of baking soda b. the percent by volume of the solution and the solubility of baking soda c. the temperature of the water and the solubility of baking soda at that temperature
Answers: 1
You know the right answer?
Show the calculation of the freezing point of a solution made by dissolving 12.6 grams of the electr...
Questions

Mathematics, 16.02.2021 02:20

Mathematics, 16.02.2021 02:20



Mathematics, 16.02.2021 02:20


Mathematics, 16.02.2021 02:20

Spanish, 16.02.2021 02:20

Mathematics, 16.02.2021 02:20



Mathematics, 16.02.2021 02:20

Social Studies, 16.02.2021 02:20



Mathematics, 16.02.2021 02:20

Arts, 16.02.2021 02:20


Biology, 16.02.2021 02:20

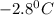
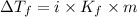
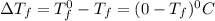 = Depression in freezing point
= Depression in freezing point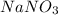 which dissociates to give two ions )
which dissociates to give two ions )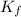 = freezing point constant =
= freezing point constant =