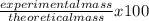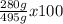Chemistry, 12.01.2020 02:31 jakobrobinette
What is the % yield when 140.0 grams of ethylene gas (c2h4) reacts with excess chlorine to form 280.0 grams of 1,2-dichloro ethane (c2h4cl2)? (given equation is c2h4 + cl2 —> c2h4cl2

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 12:00
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1

Chemistry, 22.06.2019 12:10
Building glycogen from glucose molecules is an example of
Answers: 3

Chemistry, 22.06.2019 23:40
What energy conversion occurs when a sling shot is used to shoot a rock across the room? (2 points) question 2 options: 1) stored mechanical energy is converted to mechanical energy. 2) stored mechanical energy is converted to radiant energy. 3) gravitational energy is converted to radiant energy. 4) gravitational energy is converted to mechanical energy.
Answers: 1

You know the right answer?
What is the % yield when 140.0 grams of ethylene gas (c2h4) reacts with excess chlorine to form 280....
Questions

Social Studies, 15.03.2022 21:50


Computers and Technology, 15.03.2022 21:50




Mathematics, 15.03.2022 21:50

Mathematics, 15.03.2022 21:50




Physics, 15.03.2022 21:50


Computers and Technology, 15.03.2022 22:00

Advanced Placement (AP), 15.03.2022 22:00



English, 15.03.2022 22:00